Local vibrational modes are the characteristic vibrational modes of the atoms in the defect. If I.R. light is incident on the defect atoms of the same frequency as this vibration then there is absorption. Therefore if the sample is exposed to a range of frequencies of I.R. radiation, a characteristic absorption spectrum is obtained. A defect will normally have several different absorption peaks corresponding to different types of vibration (shown below).
Another investigative tool is the use of isotopes. If the defect atom is replaced with an isotope of itself, it will now have a different mass, and therefore will vibrate at a different frequency. Therefore any absorption peaks associated with that atom will shift in the absorption spectrum.
We are able to calculate the local vibrational modes associated with our simulated defect, with any isotopic mass. These values can be directly compared to experiment; a close match is a good indication that the model is correct. There are also many other properties of the defect which it is possible for us to calculate and compare with experiment, and these are used in combination to test out a particular model of a defect.
There are various types of modes that occur, and a few xmol animations can show these. The molecule used in this example is Nickel Carbonyl, Ni(CO)4. The symmetry of the molecule means that several of the modes will be degenerate (symmetrically equivilent), and these have not been repeated.
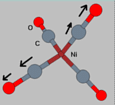 1. A symmetric breathing
mode. This is called a stretch mode, since the atoms are
vibrating along the bonds, `stretching' them. Since this mode is a
breathing mode, the molecule retains its tetrahedral symmetry
throughout, and there is no induced dipole. Therefore this mode will
be I.R. inactive, and will only show up in Raman spectroscopy. It is a
symmetric stretch mode since if more than one atom in the same bond
are vibrating, the vibration is in-phase.
1. A symmetric breathing
mode. This is called a stretch mode, since the atoms are
vibrating along the bonds, `stretching' them. Since this mode is a
breathing mode, the molecule retains its tetrahedral symmetry
throughout, and there is no induced dipole. Therefore this mode will
be I.R. inactive, and will only show up in Raman spectroscopy. It is a
symmetric stretch mode since if more than one atom in the same bond
are vibrating, the vibration is in-phase.
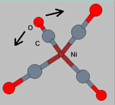 2. A wag
mode. This mode is called a wag mode since the atoms `wag' in a
direction perpendicular to the bond direction.
2. A wag
mode. This mode is called a wag mode since the atoms `wag' in a
direction perpendicular to the bond direction.
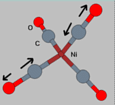 3. Anti-symmetric stretch
mode. This is anti-symmetric since there are two atoms stretching
along the bond direction but they are out of phase with each
other. Anti-symmetric stretch modes are generally of a higher
frequency than symmetric stretch modes.
3. Anti-symmetric stretch
mode. This is anti-symmetric since there are two atoms stretching
along the bond direction but they are out of phase with each
other. Anti-symmetric stretch modes are generally of a higher
frequency than symmetric stretch modes.
 4. In more complicated defects it is possible to get
combination modes, where depending on the potential in which the atoms
sit, there can be many atoms vibrating in several different
directions.
4. In more complicated defects it is possible to get
combination modes, where depending on the potential in which the atoms
sit, there can be many atoms vibrating in several different
directions.
There are actually twenty one vibrational modes of nickel carbonyl, which fall into nine distinct types; if you've never seen a molecule doing aerobics before then have a look at these!
 Directory Tree Directory Tree |
 Current
Projects Current
Projects |