


Next: Au and Ag hydrogen
Up: The electronic properties of
Previous: Introduction
Calculations were carried out for the standard defect Ci, and Ci-P,
the T-centre (Cs-CiH),
H at a bond centred site (H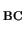 ), H at anti-bonded lattice site,
H
), H at anti-bonded lattice site,
H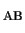 ,
VO, VP, V2, Au, Ag, Pt, Pd,
AuHn, AgHn, PdHn and PtHn and the results are given in Table 1
where they are
compared with experimental values.
,
VO, VP, V2, Au, Ag, Pt, Pd,
AuHn, AgHn, PdHn and PtHn and the results are given in Table 1
where they are
compared with experimental values.
In general the calculated electrical levels of light impurities
are within about 0.2 eV of the experimental results where available.
Whereas the donor and acceptor levels of Cs-CiH, CiP, VP, VO and even
V2
are within 0.2 eV of the experimental values, it is surprising that the
0/+ level of H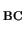 is found so close to the observed level given that
the
theory is expected to be worse when the level moves far away from
that of Ci.
For Ag, Au, Pd and Pt, all in low spin configurations, we find the
(-/0) and (0/+) levels to be deep in the gap and within 0.2 eV of
the observed values.
We now investigate the effect of adding H atoms to these defects.
is found so close to the observed level given that
the
theory is expected to be worse when the level moves far away from
that of Ci.
For Ag, Au, Pd and Pt, all in low spin configurations, we find the
(-/0) and (0/+) levels to be deep in the gap and within 0.2 eV of
the observed values.
We now investigate the effect of adding H atoms to these defects.
Table 1:
Electrical levels, eV, of deep centres. (0/+) is referred
to Ev
and
(-/0) to Ec. Tentative assignments are indicated by ?.
| Defect |
E(0/+) Calc. |
E(0/+) Observed |
E(-/0)
Calc. |
E(-/0) Observed |
Ref. |
| VP |
0.2 |
- |
0.45 |
0.43 |
|
| VO |
0.0 |
- |
0.13 |
0.18 |
|
| V2 (C2h) |
0.42 |
0.23 |
0.51 |
0.43 |
|
| C-CH |
0.24 |
? |
0.20 |
0.20 |
|
| CiP |
0.36 |
0.48 |
0.60 |
0.38 |
|
H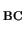 |
0.94 |
1.0 |
- |
- |
|
H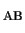 |
- |
- |
0.78 |
? |
|
| V2O |
- |
- |
0.47 |
- |
|
| Ag |
0.46 |
0.37 |
0.60 |
0.56 |
ref. [#!icds97-2!#] |
| AgH |
0.36 |
0.28 (H2) ? |
0.45 |
0.45 (E2) ? |
ref. [#!icds97-2!#] |
| AgH2 |
0.33 |
0.38 (H3)? |
0.50 |
- |
ref. [#!icds97-2!#] |
| AgH3 |
0.0 |
- |
0.13 |
- |
|
| AgH4 |
0.0 |
- |
0.97 |
- |
|
| Au |
0.21 |
0.35 |
0.66 |
0.54 |
ref. [#!svein-95!#] |
| AuH |
0.36 |
0.21 (G2)? |
0.62 |
 0.53 (G4)?
0.53 (G4)? |
ref.
[#!svein-95!#] |
| AuH2 |
0.28 |
0.47 (G3) ? |
0.62 |
- |
ref. [#!svein-95!#] |
| AuH3 |
0.0 |
- |
0.26 |
- |
|
| AuH4 |
- |
- |
1.4 |
- |
|
| Pd |
0.52 |
0.31 |
0.22 |
0.23 |
ref. [#!sachse3-97!#] |
| PdH |
0.53 |
0.55 (H280)? |
0.39 |
0.29 (E160) ? |
ref. [#!sachse3-97!#] |
| PdH2 |
0.0 |
- |
0.39 |
0.43 (E200) ? |
ref. [#!sachse3-97!#] |
| PdH3 |
0.0 |
- |
1.0 |
1.0 (H140) ? |
ref. [#!sachse3-97!#] |
| PdH4 |
- |
- |
0.53 |
- |
|
| Pt |
0.23 |
0.35 |
0.36 |
0.23 |
ref. [#!sachse2-97!#] |
| PtH |
0.52 |
0.4 (H210) ? |
0.42 |
- |
ref. [#!sachse2-97!#] |
| PtH2 |
0.0 |
- |
0.45 |
0.50 (E250)? |
|
| PtH3 |
0.0 |
? |
1.4 |
0.9 (H150) |
ref. [#!sachse2-97!#] |
| PtH4 |
- |
- |
1.0 |
- |
|
|
Table 2:
(-/-) levels of deep centres, relative to Ec,
in eV.
Tentative assignments are indicated by ?.
| Defect |
E(-/-) Calc. |
E(-/-) Obs. |
Ref. |
| V2 |
0.35 |
0.23 ? |
|
| AgH |
0.36 |
0.09 (E3) ? |
ref. [#!icds97-2!#] |
| AuH |
0.22 |
0.19 (G1) ? |
ref. [#!davidson-96!#] |
| AgH2 |
0.0 |
- |
|
| AuH2 |
0.0 |
- |
|
| PdH |
 0.0
0.0 |
- |
|
| PdH2 |
 0.0
0.0 |
- |
|
| PtH |
0.12 |
- |
|
| PtH3 |
0.0 |
- |
|
|



Next: Au and Ag hydrogen
Up: The electronic properties of
Previous: Introduction
Antonio Resende
1998-06-12
![]() ), H at anti-bonded lattice site,
H
), H at anti-bonded lattice site,
H![]() ,
VO, VP, V2, Au, Ag, Pt, Pd,
AuHn, AgHn, PdHn and PtHn and the results are given in Table 1
where they are
compared with experimental values.
,
VO, VP, V2, Au, Ag, Pt, Pd,
AuHn, AgHn, PdHn and PtHn and the results are given in Table 1
where they are
compared with experimental values.
![]() is found so close to the observed level given that
the
theory is expected to be worse when the level moves far away from
that of Ci.
For Ag, Au, Pd and Pt, all in low spin configurations, we find the
(-/0) and (0/+) levels to be deep in the gap and within 0.2 eV of
the observed values.
We now investigate the effect of adding H atoms to these defects.
is found so close to the observed level given that
the
theory is expected to be worse when the level moves far away from
that of Ci.
For Ag, Au, Pd and Pt, all in low spin configurations, we find the
(-/0) and (0/+) levels to be deep in the gap and within 0.2 eV of
the observed values.
We now investigate the effect of adding H atoms to these defects.