The first is due to Born and Oppenheimer who argue that, since the
nuclear masses are so much larger than those of the electrons, then we
can treat the nuclear motion classically and reduce the
Schrödinger equation to one only involving the electrons moving
in a potential of fixed nuclear sites denoted by R. The
solution of this equation is the structural potential energy
E(R). To determine dynamical quantities like
vibrational frequencies and diffusion barriers, we need then to solve
the classical problem of the nuclei moving in the force field ![]() .
.
Let us now consider the Schrödinger equation of the electrons in
a field of frozen nuclei. This equation still cannot be solved
exactly because of the inter-electronic Coulomb terms, ![]() . If these terms were neglected, then the Schrödinger equation
is separable and the exact wavefunction can be written down as a sum
of product orbitals
. If these terms were neglected, then the Schrödinger equation
is separable and the exact wavefunction can be written down as a sum
of product orbitals ![]() where
where ![]() are the eigenfunctions of the one-electron Hamiltonian. Of course, we
must require that the total wavefunction is antisymmetric and this can
be achieved by a suitable sum over the product orbitals. For, example
for the H
are the eigenfunctions of the one-electron Hamiltonian. Of course, we
must require that the total wavefunction is antisymmetric and this can
be achieved by a suitable sum over the product orbitals. For, example
for the H ![]() molecule, the
antisymmetric wavefunction is
molecule, the
antisymmetric wavefunction is
![]()
This type of
wavefunction is called a Slater determinant and is specified by the
electronic configuration, ie. a listing of the orbitals ![]() occupied by the N electrons.
occupied by the N electrons.
The neglect of the electron-electron interaction term is too extreme
to make this theory useful and various attempts have been made to take
this interaction into account. There are two principal schemes:
Hartree-Fock (HF) and density functional theory (DFT). Both of these
replace the electrostatic potential acting on electron i by the
other electrons, ie. ![]() by an
average over the positions of the other electrons. Thus this potential
becomes
by an
average over the positions of the other electrons. Thus this potential
becomes ![]() . Clearly the
Schrödinger equation is then separable and we can write the
wavefunction as a Slater determinant. In these theories, the
effective potential is composed of two terms: the Hartree potential
. Clearly the
Schrödinger equation is then separable and we can write the
wavefunction as a Slater determinant. In these theories, the
effective potential is composed of two terms: the Hartree potential
![]() and the exchange-correlation potential
and the exchange-correlation potential ![]() . The former is the electrostatic potential due to the electron
density of charge n(r) at point r. The density
is determined by the probability of finding a electron at the point,
or
. The former is the electrostatic potential due to the electron
density of charge n(r) at point r. The density
is determined by the probability of finding a electron at the point,
or
![]()
Here, the sum is over the occupied orbitals.
The exchange-correlation potential differs in the two theories. In HF,
it is a complicated function determined by all the orbitals ![]() . In DFT, it is rigorously determined only by the
electron density. This is an important result but the precise
dependence on density is not known except for a particular problem:
the homogeneous electron gas. In this case, the equations have been
solved numerically and the dependence of
. In DFT, it is rigorously determined only by the
electron density. This is an important result but the precise
dependence on density is not known except for a particular problem:
the homogeneous electron gas. In this case, the equations have been
solved numerically and the dependence of ![]() on
n is known exactly. For any other problem where the electron
density varies throughout space, we assume that
on
n is known exactly. For any other problem where the electron
density varies throughout space, we assume that ![]() at point r is given by the homogeneous electron gas value
involving the density n(r) at the same point r.
This is called local density functional (LDF) theory. For high
electron densities, it can be shown that
at point r is given by the homogeneous electron gas value
involving the density n(r) at the same point r.
This is called local density functional (LDF) theory. For high
electron densities, it can be shown that ![]() is
proportional to
is
proportional to ![]() and thus in LDF theory
and thus in LDF theory
![]() is also proportional to
is also proportional to ![]() . For
lower densities,
. For
lower densities, ![]() can still be written
down but is given by a more complicated expression
can still be written
down but is given by a more complicated expression
AIMPRO uses LDF theory and thus the electron density is a quantity of fundamental significance.
The problem has now been reduced to a one-electron one involving the
interaction of the electron with all the ions together with ![]() . Not all the electrons need be considered.
Electrons are divided into two groups: core and valence electrons. The
former are bound close to the ions and do not play a part in
bonding. It is highly advantageous to remove these from the
theory. This can be done through the use of the pseudopotential. For
example, in Si the 1s, 2s, and 2p electrons are
regarded as part of the core while the 3s and 3p
electrons are part of the valence shell. The four bonds associated
with each Si atom in the bulk arise from these four valence electrons.
The pseudopotential is constructed by insisting that it has exactly
the same valence energy levels as the true atomic potential,
ie. the 3s and 3p and even the 3d levels
are the same (the 3d levels are required because these orbitals
are occupied to some extent in the solid). Moreover, the
corresponding pseudo-wavefunctions are exactly equal to the true
wavefunctions outside a core whose size depends on the identity of the
atom. Inside the core, the pseudo-wavefunctions are not equal to the
true valence ones but are very smooth nodeless functions which are
easy to represent mathematically. The true 3s and 3p
wavefunctions of Si oscillate inside the core and are rather difficult
to represent mathematically. Fig. 1 shows these functions for Ni.
. Not all the electrons need be considered.
Electrons are divided into two groups: core and valence electrons. The
former are bound close to the ions and do not play a part in
bonding. It is highly advantageous to remove these from the
theory. This can be done through the use of the pseudopotential. For
example, in Si the 1s, 2s, and 2p electrons are
regarded as part of the core while the 3s and 3p
electrons are part of the valence shell. The four bonds associated
with each Si atom in the bulk arise from these four valence electrons.
The pseudopotential is constructed by insisting that it has exactly
the same valence energy levels as the true atomic potential,
ie. the 3s and 3p and even the 3d levels
are the same (the 3d levels are required because these orbitals
are occupied to some extent in the solid). Moreover, the
corresponding pseudo-wavefunctions are exactly equal to the true
wavefunctions outside a core whose size depends on the identity of the
atom. Inside the core, the pseudo-wavefunctions are not equal to the
true valence ones but are very smooth nodeless functions which are
easy to represent mathematically. The true 3s and 3p
wavefunctions of Si oscillate inside the core and are rather difficult
to represent mathematically. Fig. 1 shows these functions for Ni.
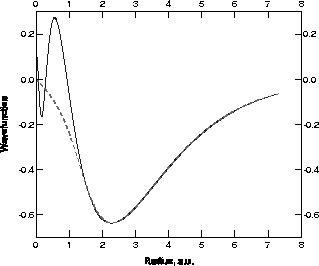
The crucial point is that the pseudopotentials have no core states at all! The lowest bound state solutions are the valence energy eigenvalues and, as stated above, these are the same as the true valence energy levels. The pseudopotential has the great virtue of making the treatment of Ge say no more difficult than carbon. AIMPRO uses the pseudopotentials due to Bachelet, Hamann and Schlüter (BHS) who have given tables for all the elements from H to Pu.
Despite these great simplifications, we are still confronted with a formidable problem. We need to solve the eigenvalue problem:
![]()
Here H includes the kinetic energy, the sum of the
pseudopotentials for all the ions present, and the effective potential
![]() which depends on the density of electrons
n(r). To proceed we expand the wavefunction in terms of
a basis
which depends on the density of electrons
n(r). To proceed we expand the wavefunction in terms of
a basis ![]() . Thus
. Thus
![]()
Two choices of ![]() are often made: plane
waves and Cartesian Gaussian orbitals. Using the first is equivalent
to making a Fourier transform of the wavefunction. This has problems
with certain elements, eg. first row, transition and
rare-earth ones where the wavefunction varies rapidly and a great
number of plane waves are required. AIMPRO uses Cartesian Gaussian
orbitals of the form
are often made: plane
waves and Cartesian Gaussian orbitals. Using the first is equivalent
to making a Fourier transform of the wavefunction. This has problems
with certain elements, eg. first row, transition and
rare-earth ones where the wavefunction varies rapidly and a great
number of plane waves are required. AIMPRO uses Cartesian Gaussian
orbitals of the form
![]()
when centred at the origin.
In practice, they are centred at the nuclei and sometimes at bond
centres sites between the nuclei. If ![]() then the
orbitals are simply spherically symmetric Gaussian functions sometimes
called s-Gaussian orbitals. If one of the
then the
orbitals are simply spherically symmetric Gaussian functions sometimes
called s-Gaussian orbitals. If one of the ![]() is unity, and the others zero, then the functions are
p-Gaussian orbitals while if the sum of the
is unity, and the others zero, then the functions are
p-Gaussian orbitals while if the sum of the ![]() is 2, the set of six orbitals generate five d-orbitals and one
s orbital. The great advantage of Gaussian orbitals is that
analytical expressions can be given for all the integrals.
is 2, the set of six orbitals generate five d-orbitals and one
s orbital. The great advantage of Gaussian orbitals is that
analytical expressions can be given for all the integrals.
We now have to solve the equations
![]()
The next step
is to multiple this equation through by ![]() and
integrate over r. This reduces the differential equation to a
set of matrix equations:
and
integrate over r. This reduces the differential equation to a
set of matrix equations:
![]()
which can be solved by
matrix methods. ![]() and
and ![]() are matrix elements of the Hamiltonian and the overlap respectively.
are matrix elements of the Hamiltonian and the overlap respectively.
Once this equation has been solved we can generate the output charge density
![]()
In general this is not equal to the charge
density used to generate the effective potential ![]() . Of course, this is wrong and we need to devise a scheme whereby the
input charge density, which is used to construct
. Of course, this is wrong and we need to devise a scheme whereby the
input charge density, which is used to construct ![]() , is equal to the output density. This is usually carried out by an
iterative procedure and the process of achieving equality in the
densities is called the self-consistent cycle.
, is equal to the output density. This is usually carried out by an
iterative procedure and the process of achieving equality in the
densities is called the self-consistent cycle.
AIMPRO uses an internal representation of the charge density by
expanding it in a basis of s-Gaussian functions ![]() . Thus
. Thus
![]()
The particular exponents of the Gaussian ![]() together with the exponents on the basis functions
together with the exponents on the basis functions
![]() have been calculated for atoms and given in a single
data file called bhs.lib.
have been calculated for atoms and given in a single
data file called bhs.lib.
Once the self-consistent density has been found, the structural energy
E(R) can be evaluated. We then compute he forces on
each atom from ![]() and move the atoms
until this energy is a minimum and these forces vanish. This is called
relaxing the assembly of atoms.
and move the atoms
until this energy is a minimum and these forces vanish. This is called
relaxing the assembly of atoms.
The user of AIMPRO needs to provide 1) the positions of the atoms in
the cluster (in atomic units); 2) the basis to be used for the
wavefunction and charge density; 3) the electronic configuration; 4)
the identities of the atoms which are to be relaxed. The output gives
the energy, electronic levels ![]() , and positions of the
atoms. More specialised output is available.
, and positions of the
atoms. More specialised output is available.